Overcoming Obstacles to Vaccines for AIDS, Tuberculosis, and Cervical Cancer
(June 2006) The development of vaccines for some of the world’s most deadly diseases—such as AIDS, tuberculosis (TB), and cervical cancer—are hampered by the complex natures of the diseases as well as the costly and lengthy process of vaccine development. Although even a partially effective vaccine for HIV or TB is likely to avert tens of millions of new infections, future vaccines for HIV or TB remain at least 10 years away. (See table for the estimated burden of disease since 2002 from AIDS, TB, and cervical cancer. For more information on barriers to research and development for an HIV or TB vaccine, see Obstacles to Vaccines for AIDS, Tuberculosis, and Cervical Cancer.)
Estimated Burden of Disease From AIDS, TB, and Cervical Cancer, 2002-2005
|
|
AIDS
|
TB
|
Cervical Cancer
|
|---|---|---|---|
| Annual deaths |
3.1 million
|
1.7 million
|
274,000
|
| DALYs lost |
84 million
|
35 million
|
3.3 million
|
Note: Disability-adjusted life-year (DALY) is a summary measure that includes the number of healthy years of life lost to premature death and the number of years spent with less than full health.
Sources: UNAIDS/WHO, AIDS Epidemic Update: December 2005 (2005); WHO, Global Tuberculosis Control—Surveillance, Planning, and Financing, WHO Report 2006 (2006); International Agency for Research on Cancer, Globocan 2002 Database (www-dep.iarc.fr); and WHO, Global Burden of Disease Estimates for 2002 (www.who.int).
And the challenges would not end once new vaccines have been developed. For instance, while two vaccines against human papillomavirus (HPV, the virus which causes cervical cancer) are almost ready for public use, their introduction into developing countries will involve overcoming issues of acceptability, delivery, and affordability, compounded by a lack of public awareness. Some of the issues confronting the introduction of the HPV vaccines would also arise in the future with an HIV or TB vaccine, so it behooves policymakers and public health officials to study these issues closely.1
Additionally, the lack of guaranteed markets in developing countries has hindered private-sector investment in vaccine development. But novel approaches to financing vaccine investment and delivering HPV vaccines to adolescents (a key target group for all three diseases) have given analysts hope that new vaccines can be developed and introduced in developing countries.
Learning From HPV Vaccine Introduction
On June 8, 2006, the U.S. Food and Drug Administration issued approval for Gardasil, the first vaccine designed to prevent cervical cancer. Approval for Gardasil was also issued in Mexico on June 1, and a vaccine may be licensed for use in some developing countries by 2007.2 But introducing the vaccine at the community level will still entail a number of challenges—the first of which will be communicating the public health benefit of an HPV vaccine.3
That HPV is a leading cause of cervical cancer has only been clarified relatively recently. And in many areas of the developing world, health care providers and policymakers (not to mention the general public) are still not fully aware of the link and therefore may not understand the public health benefits of a vaccine for preventing HPV infection. A lack of country-specific data on incidence and prevalence of HPV and cervical cancer further contributes to this low level of awareness.4
Issues of Social and Cultural Acceptability
The biggest challenge, however, lies in reaching the target population to receive the vaccine—young people. For the vaccine to be effective in preventing HPV, it should be administered before the onset of sexual activity. Vaccinating young people against an STI is likely to bring up issues of social and cultural acceptability and create reluctance among parents, communities, health providers, and political leaders.5
Some opponents in the United States have already voiced concern that delivering an HPV vaccine to adolescents would contradict messages about abstinence, while others who were initially opposed to an HPV vaccine now seem to be more receptive. Similar arguments are likely to surface in developing countries where issues of teenage sex and abstinence are already very sensitive.6 Because a future vaccine for HIV would also need to be administered before the onset of sexual activity, these issues of acceptability would also apply to an HIV vaccine.
Just Girls, or Boys, Too?
Public health officials also have to determine whether to vaccinate adolescent girls only or whether to vaccinate boys as well. Because cervical cancer affects only women, much of the emphasis for HPV vaccination has also been on women alone. In keeping with the name of their vaccine (Cervarix), GlaxoSmithKline is testing it among only women.
Meanwhile, Merck is testing Gardasil among both adolescent girls and boys, using the rationale that HPV is also responsible for considerable disease burden in men. Vaccinating men would not only decrease men’s risk of acquiring HPV, but also contribute to lower rates of HPV transmission, potentially lowering cervical cancer rates among women.7
Additional Obstacles to Reaching Adolescents
Finally, vaccinating young people involves a number of logistical barriers. The HPV vaccine is administered in three doses, which means vaccination efforts will have to reach the same young people on more than one occasion. But unlike childhood vaccination programs, the infrastructure for administering vaccines to adolescents is lacking in developing countries. Young people do not come into regular contact with the health system, which makes routine vaccination efforts particularly difficult.
Furthermore, the effectiveness of the vaccine is approximately four years, meaning that booster shots would be necessary.8 Because a future HIV or TB vaccine would also be targeted at adolescents, the challenge of being able to reach young people would also apply for an HIV or TB vaccine. The high price($120 per dose of Gardasil) would also be a limiting factor for use in developing countries—a barrier that would also apply to an HIV or TB vaccine.9
Which Comes First—Investment or Markets?
The process of developing new vaccines is exceptionally lengthy, challenging, and expensive. But several global initiatives are already underway to speed development of an HIV or TB vaccine: The International AIDS Vaccine Initiative (IAVI), the Global HIV Vaccine Enterprise, and the Aeras Global TB Vaccine Foundation.10 Encouragingly, annual public-sector investments into HIV vaccine research and development also doubled between 2000 and 2005, from $307 million to $617 million.11 And a number of organizations, including PATH and WHO, are now preparing for the introduction of Cervarix and Gardasil in developing countries.12
But despite these initiatives, funding gaps for HIV, TB, and cervical cancer vaccines still remain—caused by the high costs of development and distribution. Because expensive vaccines would be largely out of reach for developing countries, and because the pharmaceutical industry is uncertain whether it will be able to recoup its costs, pharmaceutical companies have not been willing to invest the necessary amounts to adequately support development and production costs.
One new proposal to encourage private-sector investment is an advance market commitment. In this arrangement, donors make a binding commitment to purchase a vaccine if and when it is developed. In such an agreement, governments in developing countries would decide to buy a vaccine at a low price, and then donors would commit to providing the additional money to reach a guaranteed price. Once the contracted number of vaccines has been purchased, the supplier would commit to selling additional treatments at an affordable price.13
With a guaranteed market, pharmaceutical companies would have the necessary incentives to invest in vaccine development for diseases (such as AIDS and TB) that primarily affect developing countries. Developing countries would be assured that, if a vaccine is developed, it will be rapidly available at an affordable price. By improving investment in production facilities to ensure sufficient vaccine supply at low cost, an advanced market commitment could also be beneficial for those vaccines in final stages of development such as Cervarix and Gardasil.14
The Group of Eight industrialized nations is considering an advance market commitment plan to promote development of new vaccines aimed at developing countries. According to a report by IAVI, pharmaceutical companies have responded positively to the concept of an advance market commitment, but their participation remains uncertain.15
Promising Prospects for Vaccine Introduction
Once vaccines are developed, international organizations and developing-country governments must take the lead in educating the public about the new vaccines to improve acceptability and in strengthening health systems for delivery. These tasks will be particularly important for HPV vaccines and a future HIV or TB vaccine, since their target population of young people has not traditionally been involved in immunization programs.
Interestingly, studies on acceptability have shown that, despite the objections noted above, many parents are willing to vaccinate their adolescents against HPV.16 A recent literature review on the acceptability of HPV vaccines indicates that parents, healthcare providers, and young women are interested in vaccines that prevent HPV and other sexually transmitted infections.17 Another study in the United States among parents initially opposed to HPV vaccines for their adolescents found that educational programs improved acceptability.18
Proportion of Adolescents Ages 12-14 Receiving Any Injections in the Past 12 Months, Selected Countries
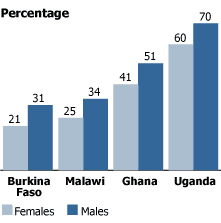
Source: A. Biddlecom, A. Bankole, and K. Patterson, “Vaccine for Cervical Cancer: Reaching Adolescents in Sub-Saharan Africa,” The Lancet 367 (2006).
A review of injection practices among adolescents in four sub-Saharan African countries also reveals encouraging prospects for introducing the HPV vaccine through existing systems. The proportion of young adolescents receiving any injections in the year preceding the survey ranged from 21 percent of girls and 31 percent of boys in Burkina Faso to 60 percent of girls and 70 percent of boys in Uganda (see figure).
Thus, HPV vaccination efforts are likely to have some level of effect even in low-resource health systems. By coordinating with school-based programs, the prospects for HPV vaccination efforts to reach a substantial proportion of adolescents are promising.19
Prevention and Treatment Remain Crucial
However, even with the introduction of new vaccines, the role of prevention and treatment programs remains crucial. “Key to success will be demonstrating effective strategies for vaccinating a large proportion of young adolescent girls—a group with very limited access to health services in many countries—and ensuring that women in their 30s and 40s are screened (and treated as necessary) at least once for cervical precancerous lesions,” says Jacqueline Sherris, PATH’s strategic program leader for reproductive health.
Finally, vaccines alone will be unable to control AIDS, TB, or cervical cancer, especially since no vaccine would be 100 percent effective. To achieve the greatest impact and to be most effective, vaccines should be accompanied by other comprehensive prevention and treatment efforts.20
Marya Khan is a research associate at the Population Reference Bureau.
References
- International AIDS Vaccine Initiative (IAVI), Vax: AIDS Vaccine Bulletin 4, no. 2 (2006).
- Merck and Company, Inc., FDA Approves Merck’s GARDASIL, The World’s First and Only Cervical Cancer Vaccine, press release (June 8, 2006), accessed online at www.merck.com, on June 12, 2006.
- Cynthia Dailard, “The Public Health Promise and Potential Pitfalls of the World’s First Cervical Cancer Vaccine,” Guttmacher Policy Review 9, no. 1 (2006); and IAVI, Vax: AIDS Vaccine Bulletin 4, no. 2 (2006).
- Program for Appropriate Technology in Health (PATH), Introducing HPV Vaccines in Developing Countries: Overcoming the Challenges, fact sheet (October 2005), accessed online at www.path.org, on March 29, 2006.
- PATH, Introducing HPV Vaccines in Developing Countries.
- Dailard, “The Public Health Promise”; and IAVI, Vax: AIDS Vaccine Bulletin 4, no. 2.
- IAVI, Vax: AIDS Vaccine Bulletin 4, no.2; and IAVI, IAVI Report 9, no. 5 (2005).
- Dailard, “The Public Health Promise”; and IAVI, Vax: AIDS Vaccine Bulletin 4, no. 2.
- Merck and Company, FDA Approves Merck’s GARDASIL, The World’s First and Only Cervical Cancer Vaccine.
- IAVI is developing vaccine candidates with its network of partners, promoting awareness among policymakers about the urgent need for a vaccine, advocating for public policies to speed vaccine development, and supporting community participation in the clinical trial process. The Global HIV Vaccine Enterprise has brought together researchers, funders, and advocates from developed and developing countries as well as the broader international community to coordinate AIDS vaccine research and development, aiming to share research successes and avoid duplication. For TB, the Aeras Global TB Vaccine Foundation is taking promising research and early vaccine candidates through preclinical testing and clinical trials to manufacturing and release, with the goal of licensing affordable TB vaccines that will be made available to people in developing countries. TB vaccine research and development has also received a boost from the Stop TB Partnership as well as the Bill & Melinda Gates Foundation, which recently tripled its investment from $300 million to $900 million for improved TB prevention and treatment tools, including a new TB vaccine. See Heidi Worley, “Intersecting Epidemics: Tuberculosis and HIV” (April 2006), accessed online at www.prb.org.
- HIV Vaccines and Microbicides Resource Tracking Working Group, Tracking Funding for Preventive HIV Vaccine Research & Development: Estimates of Annual Investments and Expenditures 2000 to 2005 (2005).
- For instance, PATH is negotiating partnerships with Merck and GlaxoSmithKline to accelerate access to HPV vaccines. In addition, PATH is examining financing options with potential funders (such as the Global Alliance for Vaccines and Immunization) to purchase HPV vaccines for public-sector programs. Harvard University is conducting policy-level impact and cost-effectiveness analyses on HPV vaccination in low-resource areas. The International Agency for Research on Cancer is working to improve the availability of country-specific data on HPV and cervical cancer. While working to facilitate the licensing of vaccines in developing countries, the World Health Organization (WHO) is also preparing to set a global policy agenda for HPV vaccine introduction. For more information, see PATH, Introducing HPV Vaccines in Developing Countries.
- Owen Barder, Making Markets for Vaccines—Ideas to Action (Washington, DC: Center for Global Development, 2005).
- Barder, Making Markets for Vaccines.
- IAVI, Vax: AIDS Vaccine Bulletin 3, no. 9 (2005).
- Eduardo Lazcano-Ponce et al., “Acceptability of a Human Papillomavirus (HPV) Trial Vaccine Among Mothers of Adolescents in Cuernavaca, Mexico,” Archives of Medical Research 32, no. 3 (2001): 243-47.
- Gregory Zimet, “Improving Adolescent Health: Focus on HPV Vaccine Acceptance,” Journal of Adolescent Health 37, no. 6 (Supplement) (2005): S17-S23.
- Kristin Davis et al., “Human Papillomavirus Vaccine Acceptability Among Parents of 10- to 15-Year-Old Adolescents,” Journal of Lower Genital Tract Disease 8, no. 3 (2004): 188-94.
- Ann Biddlecom, Akinrinola Bankole, and Kate Patterson, “Vaccine for Cervical Cancer: Reaching Adolescents in Sub-Saharan Africa,” The Lancet 367, no. 9519 (2006): 1299-1300.
- IAVI, Developing and Delivering an AIDS Vaccine: Issues and Answers, Policy Brief #1 (2004), accessed online at www.iavi.org, on May 11, 2006.
