How Fear and Loneliness Shape Body Chemistry and Health
The chronic stress of living in poverty, loneliness of social isolation, and fear endemic in some high-crime neighborhoods can alter gene activity and contribute to disease, according to Steve Cole, professor of medicine and behavioral sciences at University of California, Los Angeles (UCLA). His research examines how psychological experiences influence immune systems and human health. He is mapping the biological pathways through which social conditions change the expression of traits or tendencies encoded in genetic material.
Researchers in this growing field of “social genomics” are finding that social activities today may influence our bodies for the next few months or for the rest of our lives, he reports. But he emphasizes the following:
- Interventions early in life may provide health benefits that accrue over a lifetime.
- Even after exposure to adverse social conditions that cause health to decline, individuals placed in a more favorable environment may see their health rebound.
- Understanding at a molecular level the biological responses that promote disease will help scientists act before a disease manifests itself and produces irreversible harm.
Cole spoke with Marlene Lee, program director of academic research and relations at the Population Reference Bureau, about advances in genetics and the implications for population research. Below are excerpts from their conversation.
Lee: What kinds of social activity affect our bodies and which activities have short-term implications and which have long-term implications?
Cole: In general, the things that people worry about a lot are what we often call acute stressors—really bad events like the death of a loved one or a really frightening event, traumatic experience, or just having a terrifically bad day. It turns out that the epidemiology suggests that it is not that kind of acute stress that is most detrimental to health. What’s really detrimental to health are long-term stressful lifestyles: social behaviors that may not be terribly bad in any given day but that add up, when repeated day after day, month after month, year after year, to a life that is filled with threat and uncertainty. That threat doesn’t even have to be particularly conscious—it may just be subtly there in the background. The kinds of social and environmental variables that seem to trigger that kind of chronic “stress/threat” psychology are things like poverty, the sense of being alone or separate from humanity, or a big threat that hangs over your head for a long time like a diagnosis with a chronic disease such as cancer. Worrying constantly about whether we or the people we care about are safe—is there going to be a war or a shooting. That kind of psychology is the kind that correlates best with the risk of disease in epidemiologic studies… When we look into genomics to try to understand how diseases come about, we are starting to find that it is these chronic stressors that the genome responds to in a way that sets us up for disease down the line.
Lee: Does a heightened sense of not being safe or of insecurity among the elderly play a little bit into aging being more stressful and having long-term effects later in life?
Cole: Yes, I think it may, both in terms of people feeling like they are less able to cope with a complicated environment as they are older and also more aware, because of their greater life experience, of the kinds of things that could come upon them. That said, lots of older people are mentally skilled enough to find their way into a niche, a place in the world, where they are fundamentally comfortable. They are around people that they know and care about and that know them and care about them. That kind of capacity to control your own life and to make wise decisions, and find the things that work for you, is something that older people are often quite good at. We kind of raise our own stakes as we age. There is much more variability in people’s biology and outcomes as a result of the increasing leverage they have over their own lives and their own personal histories.
Lee: How is what you think of as the “new genetics” and the advances in genetics different from the genetics some people may have learned in high school?
Cole: …As we get the tools to watch genes in action, we have begun to recognize that they do not necessarily exert the same influence on every single individual. Whether a gene is active, or how active it is, or how much it influences your cellular physiology depends a lot on the environment you are in. That environment includes the physical and chemical environment—the things that we traditionally think of as physiological stimuli: temperature, humidity. But it can also include the social world and how we perceive the world. If we perceive it as threatening or uncertain, our bodies will mount “defensive” stress responses. They will release hormones and neurotransmitters that activate receptors on cells, and that kicks off changes in gene expression. We can start to see how psychology meets up with physical events and chemical events to jointly regulate what our genome does. We have gone beyond a static view of genes as just blocks that are handed to you by your genetic endowment from your parents. We now think of them as a template that reflects the conditions of the life that you lead back into the body in the form of changes in cellular physiology.
Lee: You and others have written about “socially sensitive” genes. …How does identification of socially sensitive genes influence understanding the relationship between social conditions and health?
Cole: What has been amazing in the last 10 or 15 years is our ability to poll the whole human genome. We have remarkable biotechnology that allows us to look at every single one of our 21,000 genes as they respond to these different kinds of social, psychological, physical, and chemical environments. That allows us to map the whole system as it is working and ask questions about which components of the system are sensitive to any given kind of stimulus or environment. …When we study people confronting a wide variety of different kinds of adversity—bereavement, social isolation, poverty, or post-traumatic stress, for example—we see a fairly recurrent pattern in which one group of genes involved in inflammatory responses becomes activated. You start to transcribe more copies of that gene DNA into messenger RNA and that builds more inflammatory proteins in your cells. That genetic program probably evolved to defend [us] against the likelihood of physical injury, which for most of our history as a species has been associated with the psychological experience of flight, threat, or stress… When we confront extended periods of threat or uncertainty, that “pro-inflammatory” group of genes involved in defending us against bacteria become more active. Conversely, genes that defend us against viral infections…become less active. As a consequence of those two things, we become both more vulnerable to viral infections but also we grow more vulnerable to a variety of different chronic diseases that are caused by the physiological side effects of those inflammatory genes that are trying to defend us against those bacterial infections… Pro-inflammatory genes also accidentally fertilize the development of atherosclerotic plaques and other forms of cardiovascular disease, the development and metastasis of cancer cells, and the degeneration of our neurons. That is an unwanted byproduct of this defensive gene expression response. You can think of [the pro-inflammatory response to stress] as a kind of double-edged sword: It kills bacteria, but it also makes it easy for other kinds of diseases to progress. What happens in heart disease is there is damage on the wall of a blood vessel and this inflammatory response tries to heal that damage, but inadvertently builds a plaque, sort of like a scar, that starts to block your blood vessels. A similar kind of defensive response gone wrong happens in neurodegenerative diseases. You have more inflammation going on than is really healthy for the neurons and so the neurons basically get sick and die by accident while the immune system is trying to defend you against tissue damage. Similar things happen with metastatic cancer. We are now finding that many tumors start to grow in our body but they can’t actually escape and metastasize (and that is what actually causes disease and death). But one thing that does help them metastasize and colonize distant tissues is when inflammation grows blood vessels into the environment. That helps cells survive longer, it provides growth signals and other kinds of resources that a renegade cancer cell can take advantage of and use to escape into the rest of the body. …This is why, if you feel stressed for years and decades, that can eventually add up to changes in the way that very slow biological processes work in your body and contribute to these chronic diseases.
Lee: Do you have additional insight into the different pathways through which social environment controls gene expression?
Cole: …We are understanding how threat, stress, fear, and anxiety get out of the brain and into the body. [They] travel through a division of our nervous system—the sympathetic nervous system, popularly called the fight-or-flight stress response. The chemicals released by those neurons in the distant parts of the body are well-known to activate certain kinds of receptors that control the activity of pro-inflammatory and anti-viral genes. In the past ten years, we have been able to map out quite clearly that when you have the fight or flight response, the neurotransmitter norepinephrine interacts with beta-adrenergic receptors and stimulates the expression of the interleukin 6 gene, which then stimulates inflammatory biology… In another stress response pathway, hormones regulate the activity of our adrenal glands, and the adrenal glands produce another set of hormones that go out into the body, in particular cortisol, to regulate gene expression in just about every tissue. So for stress and fear, we have a pretty good biochemical roadmap of how these gene expression changes take place. One of the big frontiers right now is how positive psychology gets out into the rest of the body, if it does. It is possible that positive psychology works only by reducing stress or threat responses. But a lot of people are interested in the possibility that separate from reducing threat or stress, positive psychology may have its own hormones and neurotransmitters that also influence gene expression…
Lee: Could you give an example of early social experience and what it changes in terms of gene expression?
Cole: One of the best examples comes from a line of research on monkeys conducted by Stephen Soumi. These are monkeys that are newborn. They’ve had some exposure to the world through the mother in utero, but basically they are fresh to what we would call the environment and the world outside. Dr. Suomi randomizes them to either stable social conditions where an adult [mother] is there to structure what they are doing, or to spend the first couple of months of their lives in a cage full of other infant monkeys without any adult supervision. In the absence of any stabilizing adults, that social environment is somewhat unstable and chaotic but not overtly violent or injurious. That is actually a reasonably good model of the kinds of psychological experiences that really get to us in modern society. …There’s not a lot of violence that takes place to the average person in America on a daily basis, but there’s a lot of threat and uncertainty and chaos and the need to figure out what is going on and how to confront all the random crazy things that happen to us in life. …What we have been learning is that even things that are not that extreme still trigger the same kind of psychology. Let’s say that you are in a family but you have an unusually volatile and unpredictable social scene: mom and dad don’t get along, or maybe dad’s not around very often, or maybe mom’s not around very often, or maybe there are just constantly new people coming into the house for whatever reason and you don’t understand why. All of that will leave you feeling disoriented and like the world is an unpredictable place. I need food and I cry: Sometimes I get it [food] and sometimes I don’t. To make a long story short, when life is chaotic and unpredictable (even for a few months), that seems to activate some of these defensive gene expression programs. When Dr. Soumi randomizes the monkeys to the more unstable environment, the gene expression profiles in the white blood cells of these animals look like they are under stress—they turn on that inflammatory response. What’s remarkable is that after a few months, he can take that monkey from an unstable environment and put it in with all the rest of the monkeys reared in a more-stable social system. So you have a monkey that has been in an unstable chaotic environment, and now you put all the monkeys together…. [for] the rest of their lives, the animals that spent the first few months of their lives in the chaotic environment behave differently. Moreover, they continue to show differences in gene expression, months and now years, after that initial unstable early environment is gone.
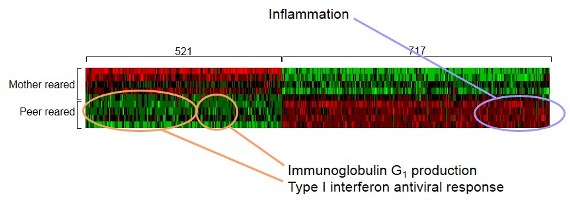
This “heat plot” shows differences in the expression of genes regulating immune system functioning between monkeys who experienced social instability (peer reared) and those who did not (mother reared)—red representing up-regulated genes and green representing down-regulated genes.
Source: Steve Cole, “Transcriptional Modulation of the Developing Immune System by Early Life Social Adversity,” Proceeding of the National Academy of Sciences of the United States of America 109, no. 50 (2012): 20578-83.
Lee: I see this as quite revolutionary in terms of how we think about the environment that we create for people in our society. What do you think are some of the implications of this new genetics and some of these types of findings for public policy or dealing with creating interventions for particular types of diseases?
Cole: The first thing is that the scenario I just outlined suggests that all other things equal, interventions that take place early in the life span are probably going to have more leverage because there is more of your life span remaining for benefit to accrue, and because young bodies are so impressionable. A second thing that comes to mind is that I don’t want to leave anybody with the impression that the die is cast when you are young and that there is nothing you can do after that. We have never seen a situation where, even in the oldest animals, there is not some plasticity, or some ability to catch up or rebound when you come into a more favorable environment. So even late in life, in mid-adulthood or late-adulthood, when people are put into more favorable environments, the genome gets the message, and it starts to respond in a more favorable way—less inflammation and more healthy antiviral responses for example in white blood cells. Even in adulthood there is room to change your world for the better and to have that filter down through your body into the genome… Just because adversity can get into the body does not mean that there is not a way to launder it out by straightening up the environment that you are in now and leading the most salutatory and favorable lifestyle for who we are temperamentally. And I think the third thing that also jumps out—the more we learn about these gene expression profiles the more we can see diseases before they even become an illness. We start to understand the ingredients of a heart attack or the ingredients of Alzheimer’s disease or the ingredients of a case of metastatic cancer. We are beginning to recognize that the same sets of genes contribute to all of these things… there are usual suspects involved in all of these things like the genes that mediate inflammatory responses… We can see disease brew for years and years before it actually rears its head as a significant impairment or overt symptoms for an individual. That is an exciting opportunity because it allows us to think about what is going on for this person before their health goes around the bend, and it is really hard to get it back. I am cautiously optimistic that as we learn more about the molecular biology and the genomics of disease, we can start to respond to adversity and its effects on the body before we get into these irreversible disease states. We can start to monitor how are we doing: Have we picked a good life for ourselves? Is the set of policies we have working well for people at the regional or state or national levels? Is this really working for people or are we still seeing bodies that look, at the genomic level, [like] they are feeling stress and threat and that we know in the end will then add up to differences in disease rates…
Lee: What is the one thing you would recommend if you could make a law or tell people what important concrete steps might be needed?
Cole: I don’t think I have a simple answer except in one regard. …I got to this research by looking at minority groups that were stigmatized to the point of being seriously threatened—having to fear for their lives if it were known that they were a member of a particular group. That seemed to be exceptionally corrosive to health and well-being. …I think it is deeply important that we create a society where every human being feels safe. Ideally we would all feel valued, but at least each of us needs to feel safe.
This interview was initiated as a follow-up to a talk given by Steve Cole at a RAND conference marking the 20th anniversary of the National Institute on Aging Centers on the Demography, Economics, and Epidemiology of Aging. Cole is professor of medicine, psychology, and behavioral sciences at UCLA and a member of the USC/UCLA Center of Biodemography and Population Health. To learn more:
Steve Cole, “Nervous System Regulation of the Cancer Genome,” Brain, Behavior and Immunity 30, Supplement (2013): S10-18.
George M. Slavich and Steve Cole, “The Emerging Field of Human Social Genomics,” Clinical Psychological Science (forthcoming, published online March 5, 2013).
Steve Cole, “Social Regulation of Human Gene Expression: Mechanisms and Implications for Public Health,” American Journal of Public Health (forthcoming).
Definitions: The human genome is a set of DNA sequences contained within cells of the human body, providing instructions about how to build these cells. A gene is one sequence of DNA that provides instructions to make a specific chemical in the body, usually a protein. Additional background: http://ghr.nlm.nih.gov/handbook/howgeneswork/makingprotein
