Mark Mather
Associate Vice President, U.S. Programs

Dementia is one of the nation’s most expensive old-age health conditions and the most time consuming for family caregivers.
May 28, 2020
Associate Vice President, U.S. Programs
Senior Writer
Dementia is one of the nation’s most expensive old-age health conditions and the most time consuming for family caregivers. As many as 6 million people ages 65 and older live with Alzheimer’s disease in the United States, representing about one in 10 older Americans.1
However, rates of dementia are not uniform across the older population. Those with lower levels of education, the oldest old (people ages 85 and older), women, and racial and ethnic minorities are at greater risk of dementia. The types of living arrangements of people with dementia—whether they live at home, in a residential care setting (such as assisted living), or a nursing facility—also differ depending on the availability of family caregivers and financial resources.
This issue of PRB’s Today’s Research on Aging (Issue 40) summarizes what we know about the characteristics of people with dementia and their caregiving and living arrangements based on studies funded by the National Institute on Aging. Understanding the characteristics of those with dementia can help lawmakers design policies that better meet the needs of this rapidly growing population and their families.
Many conditions and diseases can cause dementia—a set of symptoms that may include memory loss and difficulties with thinking, problem-solving, or language. Alzheimer’s disease is the most common cause, but dementia can also be caused by injuries from impaired blood supply to the brain, often after a stroke. Other types of dementia include Lewy body dementia and frontotemporal disorders.
Alzheimer’s disease and other related dementias are characterized by progressive cognitive decline that interferes with independent functioning. In the National Health and Aging Trends Study (NHATS)—a nationally representative sample of Medicare beneficiaries ages 65 and older—respondents are classified into three categories: those with no dementia, possible (or early stage) dementia, and probable dementia. Participants are classified as having probable dementia if a doctor has told the person that they have dementia or Alzheimer’s disease.
For respondents without a diagnosis, dementia status is determined through a test measuring cognitive functioning, including memory (word recall), orientation (such as knowing the date and year), and executive functioning (drawing a specific time on a clock). In addition, for respondents unable to self-report, proxy respondents (typically a family member) answer the AD8, a series of eight Yes/No questions about the respondent (problems with judgment, reduced interest in hobbies, repeats self, trouble using tools or appliances, forgets correct month/year, trouble handling finances, forgets appointments, daily problems with memory/thinking).2
Cut points (based on standard deviations from the mean) are then used to group study participants into different categories. In the 2011 NHATS, 11% of Medicare beneficiaries ages 65 and older were classified as having probable dementia (10% among the non-nursing home population).3
Clinical guidelines developed by the National Institutes of Health and the Alzheimer’s Association also define three stages of Alzheimer’s disease (the most common type of dementia):
People with advancing dementia (or moderately severe dementia) are more likely to have difficulty with one of three daily living activities (dressing, bathing, or using the toilet) and cognitive difficulties that make it more difficult to manage medications or finances, whereas people with advanced dementia have difficulty with all these functions and more.5
No cure for Alzheimer’s disease currently exists, and no treatments have been proven to prevent its onset or delay its progression, but researchers are studying ways to treat the disease and expand support for people with Alzheimer’s disease and their families.
Higher educational attainment is associated with a lower risk for dementia. Older adults with more education have lower prevalence of dementia, more years of cognitively healthy life, and fewer years with dementia.6 In 2015, 6% of older college graduates (ages 70 and older) had probable dementia, compared with 19% of their counterparts with less than 12 years of education (see Figure 1).
Numerous studies have found that more schooling is associated with a lower risk of dementia. Researchers explain this connection in a variety of ways. They suggest that education may directly affect brain development by creating a cognitive reserve (stronger connections among brain cells) that older adults can draw upon if their memory or reasoning ability begins to decline. They also suspect that people with more education may be better able to develop techniques to compensate or adapt in the face of disrupted mental functions. In addition, education brings multiple advantages. They point out that people with more education tend to have healthier lifestyles, higher incomes, better health care, and more social opportunities—all associated with better brain health.
The share of the older population with dementia has fallen over the past two decades and continues to decline. Analysis of data from the U.S. Health and Retirement Study (HRS) found that dementia declined from 11.6% in 2000 to 8.8% in 2012 among those ages 65 and older.7 Another study using data from NHATS found a decline from 13.0% in 2011 to 11.8% in 2015 among those ages 70 and older.8 The downward trend is likely the result of better brain health related to higher levels of education.9
Prevalence of Probable Dementia Among the U.S. Population Ages 70 and Older, 2015
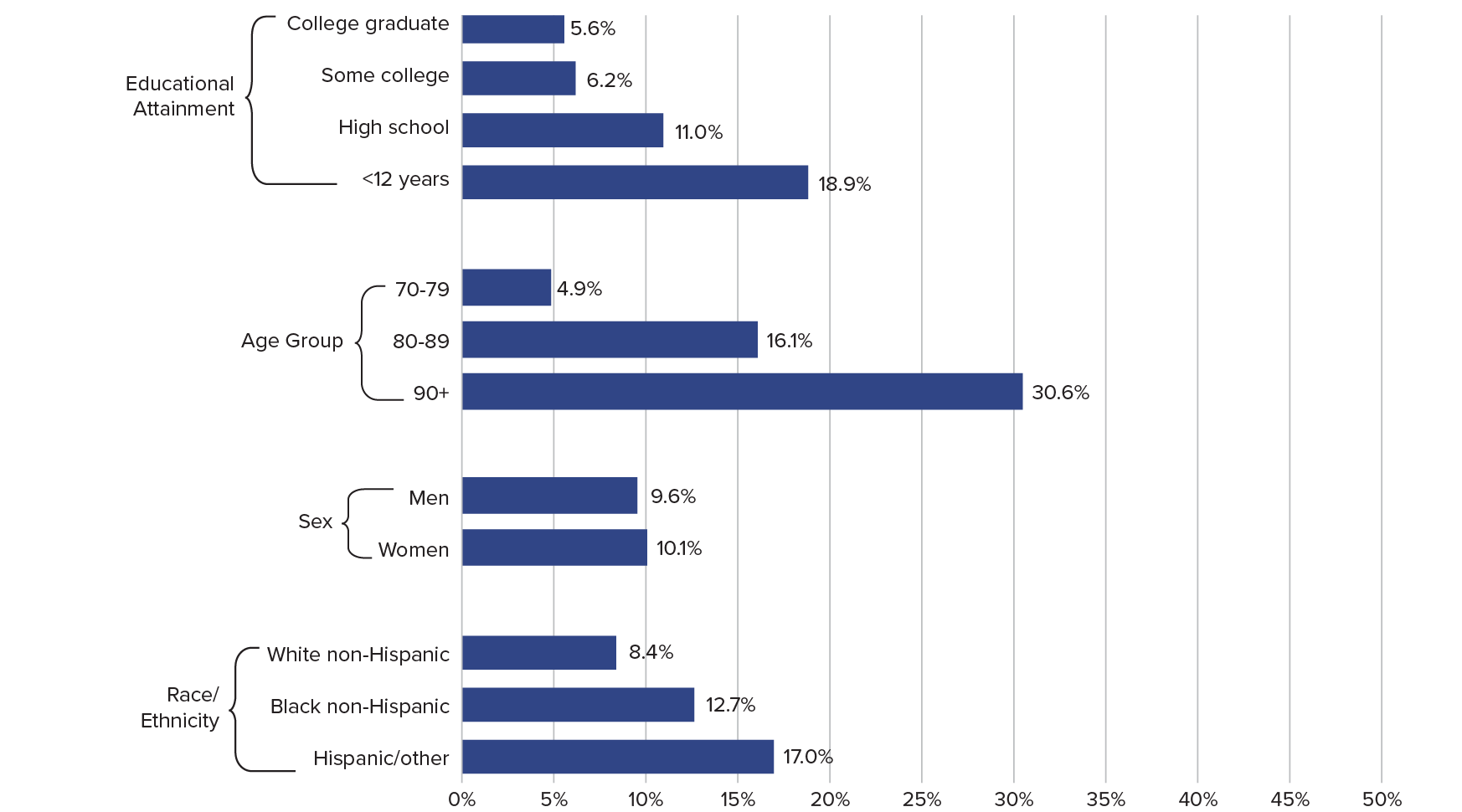
Note: Excludes persons in nursing homes.
Source: Vicki A. Freedman et al., “Short-Term Changes in the Prevalence of Probable Dementia: An Analysis of the 2011–2015 National Health and Aging Trends Study,” Journals of Gerontology, Series B 73 (2018): S48-S56.
Dementia prevalence increases with age. About 5% of adults ages 70 to 79 had probable dementia in 2015, compared with 16% of adults ages 80 to 89 and 31% of adults ages 90 and older. As the U.S. population grows older, the number of people with dementia is projected to increase sharply.10
Women are slightly more likely to have dementia than men. Among adults ages 70 and older, 10.1% of women and 9.6% of men had probable dementia.
Non-Hispanic whites have lower rates of dementia than other racial/ethnic groups. The rates of probable dementia among “Hispanic and other” racial/ethnic minorities ages 70 and older (17%) was double the rate among non-Hispanic white older adults (8%). Recent declines in dementia prevalence have been concentrated among non-Hispanic white and non-Hispanic black groups, whereas dementia prevalence has been persistently higher among the Hispanic/Latino population.11
Racial/ethnic differences in dementia prevalence are linked to immigrant status. Researchers have found that non-Hispanic white, Hispanic, and other immigrants have higher rates of dementia compared with their U.S.-born counterparts. However, the opposite is true for non-Hispanic black immigrants, who are less likely to have dementia than U.S.-born, non-Hispanic African Americans.12
Married older people may have a lower risk of dementia than their unmarried counterparts. A study that tracked a nationally representative set of HRS participants over 14 years found that married people were less likely to experience dementia as they aged.13 By contrast, unmarried older adults—including those who were cohabiting, divorced/separated, widowed, or never married—had significantly higher odds of developing dementia over the course of the study.
Divorcees were about twice as likely as married people to develop dementia, with divorced men facing a greater risk than divorced women. The researchers’ analysis showed that differing economic resources and health-related factors (such as behaviors and chronic conditions) only partly accounted for higher dementia risk among unmarried people.
The rate of Americans who die from dementia is increasing. In 2017, 66.7 deaths per 100,000 people were attributed to dementia, compared with 30.5 in 2000, according to a recent report from the U.S. Centers for Disease Control and Prevention (CDC).14 Part of this increase may reflect the decline in mortality due to other chronic conditions, such as heart disease. Since many people may go undiagnosed, published death rates may understate dementia-related deaths.
In 2017, Alabama, Georgia, Kentucky, North Carolina, South Carolina, and Tennessee were among the states with the highest age-adjusted death rates for dementia (more than 80 deaths per 100,000 population; see map). Maine, Massachusetts, and Oregon also had relatively high death rates from the disease, whereas New York had the lowest death rate for dementia (43 per 100,000).
Geographic variations across states may reflect different approaches to diagnosing dementia, different guidelines for coding dementia, and differences in the socioeconomic status and racial/ethnic composition of populations across geographic areas.15 The CDC adjusts for age differences across states to ensure that differences in death rates between geographic areas are not due to differences in the age distribution of the populations being compared.
Age-Adjusted Death Rates per 100,000 by State, 2017
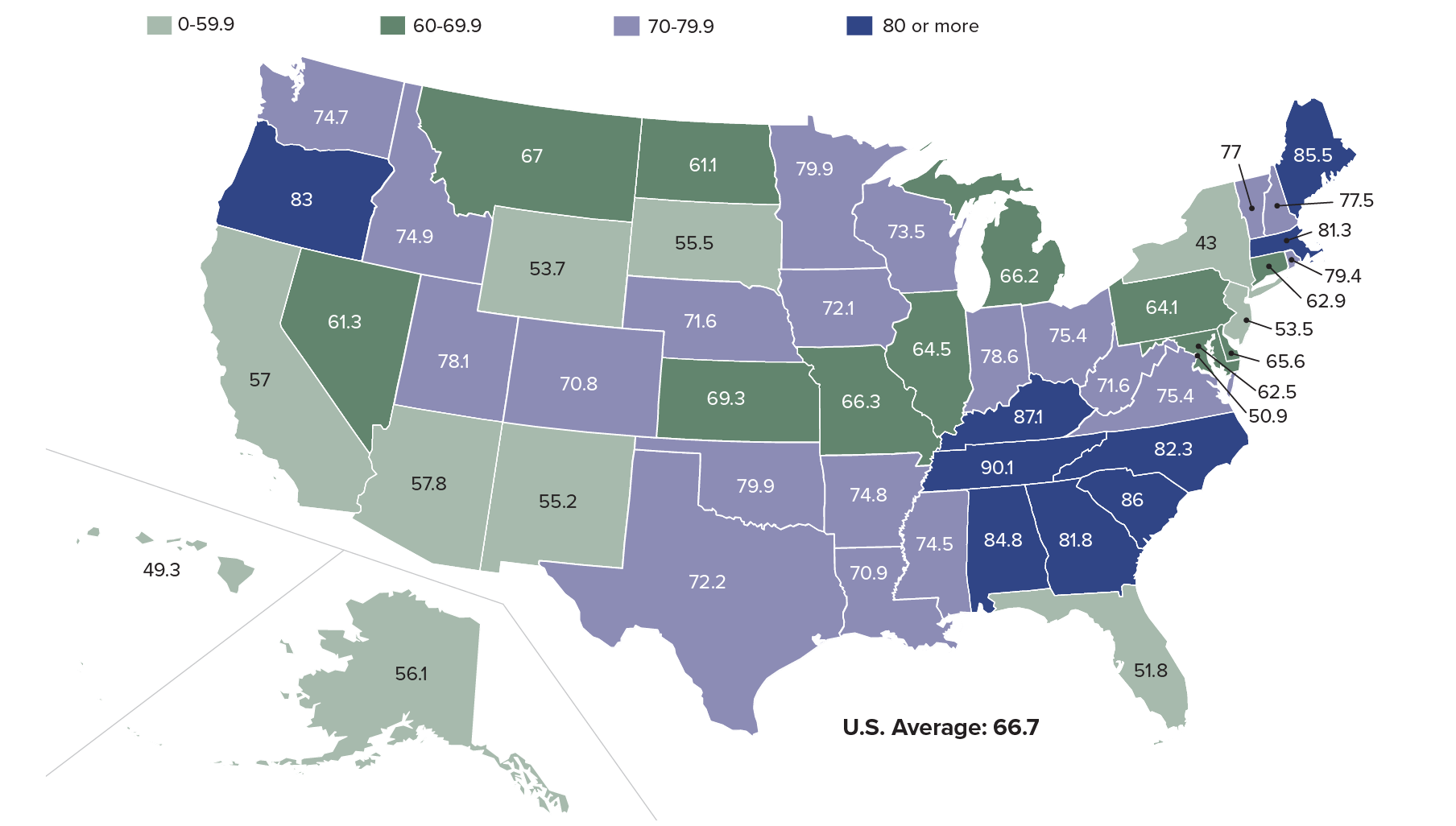
Source: Ellen A. Kramarow and Betzaida Tejada-Vera, “Dementia Mortality in the United States, 2000-2017,” National Vital Statistics Reports 68, no. 2 (2019).
| wdt_ID | States | Deaths | Rate per 100,000 |
|---|---|---|---|
| 1 | United States | 261,914.0 | 66.7 |
| 2 | Alabama | 4,815.0 | 84.8 |
| 3 | Alaska | 253.0 | 56.1 |
| 4 | Arizona | 5,045.0 | 57.8 |
| 5 | Arkansas | 2,735.0 | 74.8 |
| 6 | California | 25,017.0 | 57.0 |
| 7 | Colorado | 3,806.0 | 70.8 |
| 8 | Connecticut | 3,351.0 | 62.9 |
| 9 | Delaware | 881.0 | 65.6 |
| 10 | District of Columbia | 358.0 | 50.9 |
| 11 | Florida | 17,523.0 | 51.8 |
| 12 | Georgia | 7,659.0 | 81.8 |
| 13 | Hawaii | 1,151.0 | 49.3 |
| 14 | Idaho | 1,371.0 | 74.9 |
| 15 | Illinois | 10,147.0 | 64.5 |
| 16 | Indiana | 6,190.0 | 78.6 |
| 17 | Iowa | 3,270.0 | 72.1 |
| 18 | Kansas | 2,590.0 | 69.3 |
| 19 | Kentucky | 4,404.0 | 87.1 |
| 20 | Louisiana | 3,564.0 | 70.9 |
| 21 | Maine | 1,705.0 | 85.5 |
| 22 | Maryland | 4,403.0 | 62.5 |
| 23 | Massachusetts | 7,584.0 | 81.3 |
| 24 | Michigan | 8,523.0 | 66.2 |
| 25 | Minnesota | 5,672.0 | 79.9 |
In 2015, about 85% of Americans with probable dementia lived at home in the community or in supportive care settings (such as assisted living or personal care homes), whereas about 15% lived in nursing facilities.16 Among those with dementia living in settings other than nursing homes in 2011, 80% were in traditional community settings and about 20% lived in residential care settings (about 15% in assisted living and another 5% in independent living).17
The availability and capacity of family and other informal caregivers, income and asset levels, and the type of care needed all contribute to determining the setting in which older adults with dementia live. Eligibility for government programs and the availability of services, some of which vary by state, also play key roles.
People with advancing dementia who live at home are more likely to be racial and ethnic minorities, foreign born, and less educated. Krista Harrison and colleagues used NHATS data to examine the living arrangements of older adults with advancing dementia, defined as probable dementia that interferes with some aspects of personal care or household activities.18 Those living at home were much more likely to be black or Hispanic, not born in the United States, and lack a high school diploma—characteristics that are linked to socioeconomic disadvantage and health disparities (see Figure 2).
People with advancing dementia who live at home have more social support but also more chronic conditions. Older adults with advancing dementia residing at home tend to have more social support (a partner, larger social networks) and less functional impairment than their counterparts in residential care or nursing facilities. At the same time, home-dwelling people with dementia tend to have worse overall health and more chronic conditions.
The researchers question whether “living at home with dementia is achieved at the expense of trade-offs on family members’ mental, physical, and financial well-being, or results in disparities in unmet needs or access to high-quality dementia care.”
Most older adults with cognitive impairment who live alone rely on an adult child for assistance. Milder forms of cognitive impairment often precede a dementia diagnosis. For those with cognitive impairment residing alone, another analysis of NHATS data showed that in 2011, two in three (66%) relied on an adult son or daughter for assistance that enabled them to live independently.19 About one in eight relied on paid professionals as primary caregivers. Most of the adults with cognitive impairment were older, widowed females.
Socioeconomic and Demographic Characteristics of Medicare Enrollees Ages 65 and Older With Advancing Dementia, by Care Setting
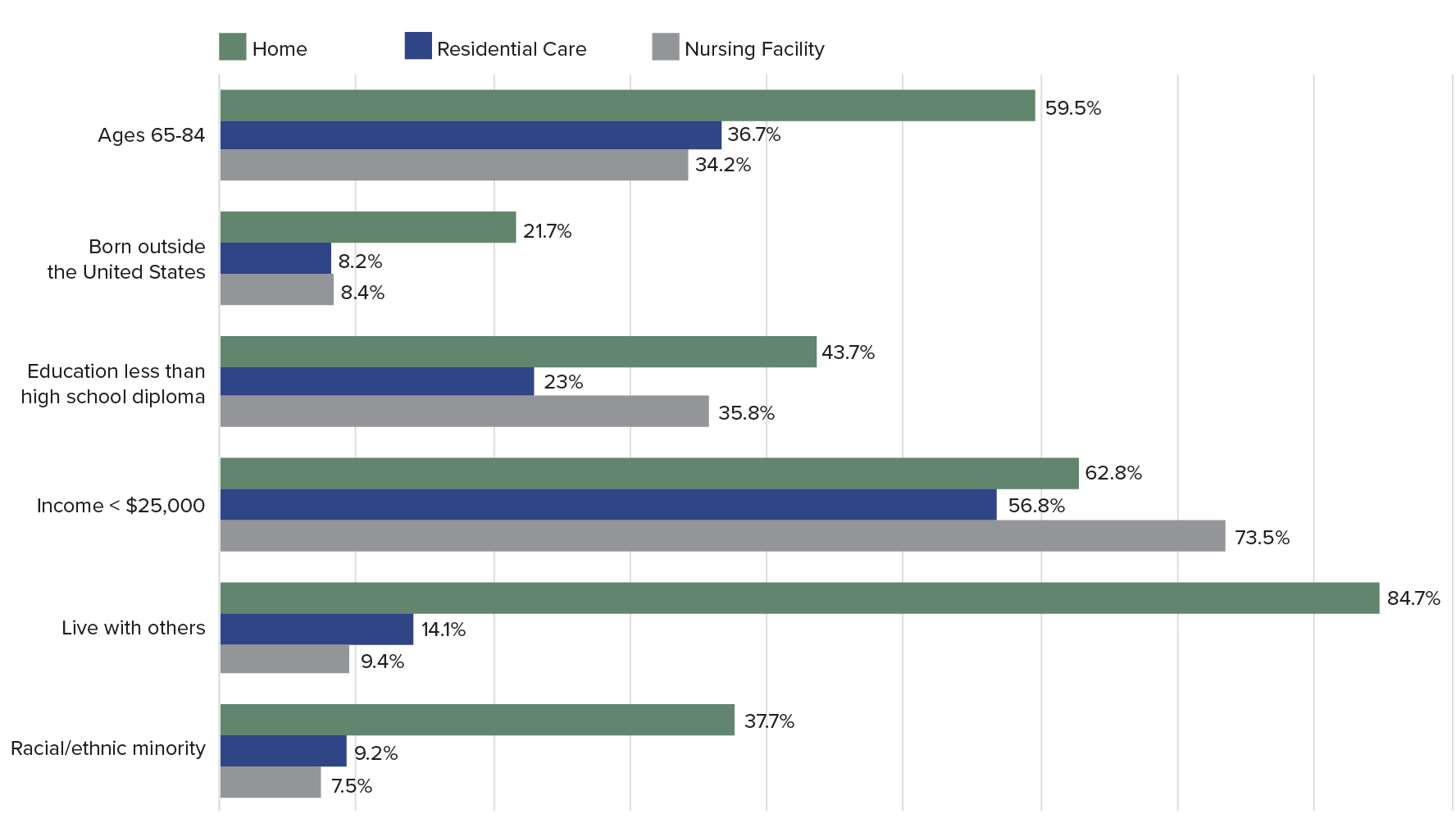
Notes: All differences comparing home versus residential care versus nursing home significant at P > .05. The amount used in many states in 2012 to determine eligibility criteria for Medicaid-paid nursing home care was $25,000.
Source: Krista L. Harrison et al., “Care Settings and Clinical Characteristics of Older Adults With Moderately Severe Dementia,” Journal of the American Geriatric Society 67, no. 9 (2019): 1907-12.
Most older people, including those with dementia and their caregivers, prefer to receive care at home rather than in nursing facilities, and many families take pride in providing care themselves. Reflecting these preferences, an increasing proportion of Medicaid funds for lower-income older adults are being spent on home- and community-based services and support (such as personal care assistance and home-delivered meals) rather than on institutional care.20 These services vary considerably by state and aim to enable people with dementia to continue living at home or in a residential care setting rather than in more costly nursing facilities.
Higher-income people with dementia are somewhat more likely to live in residential care, whereas lower-income people with dementia are more likely to live in nursing homes or at home.21 Medicare and Medicaid policies contribute to shaping these residential patterns. Medicare does not pay for most long-term support and care in either a residential care setting, such as an assisted living facility, or a nursing home. For lower-income older adults (or those who spend down their savings or assets to qualify), Medicaid may pay a portion of supportive care costs, primarily in nursing facilities, depending on the state. The median annual cost of assisted living, one type of residential care that typically provides social but not medical support, was $49,000 in 2019. The median annual cost of a nursing facility that provides medical support, which often serves lower-income older people with Medicaid coverage, was $90,000.22
Middle-income people with dementia are less likely to receive paid care. An analysis of NHATS data by Jennifer Reckrey and colleagues showed that only one in four older adults with probable dementia living at home (25.5%) received paid care in 2015.23 Among the fraction with advanced dementia, only about half (48.3%) received paid care. Analysis showed that those receiving paid care were more likely to qualify for Medicaid, be male, unmarried, have fewer children, and require more help with daily activities than their peers without paid care.
The researchers observed that older adults with probable dementia in the lowest and highest income groups (below $12,000 and above $43,000 annually) were somewhat more likely to receive paid care, reflecting that “not just functional need but also financial resources and Medicaid eligibility” contribute to patterns of where older people with dementia live.
“[T]he middle class, who neither qualify for Medicaid-funded home care nor have the means to pay for significant paid caregiving out of pocket, face a unique challenge: They must either rely solely on family caregivers to meet their needs or pay out of pocket for paid caregivers until their wealth is exhausted and they become Medicaid eligible,” the researchers write.
Dementia care is more costly than other conditions and puts a disproportionate burden on families. What people with dementia need most is supervision and help with personal care and household activities—services not covered by Medicare—whereas the drugs or surgeries commonly used to treat conditions such as heart disease and cancer are covered.
“The lifetime cost of dementia is high,” report researchers from the RAND Corporation. Using HRS data, RAND researchers found that those who live with dementia for at least six months pay, on average, $38,500 more out of pocket from age 65 to death (adjusting for length of life, demographics, lifetime earnings, and other health conditions).24 These dementia-related costs are nearly all composed of spending on nursing homes.
Similarly, Amy Kelley and colleagues used HRS data to show that health care for Medicare beneficiaries in the last five years of life was far more costly and involved significantly higher uncovered out-of-pocket costs for those with dementia than for those with heart disease, cancer, or other medical conditions.25 Out-of-pocket costs averaged $62,000 for people with dementia, more than 80% higher than for someone with heart disease or cancer.
Families of older people with dementia also spent a larger share of family assets for care in the last five years of life than families of those with other conditions. During these years, families spent nearly a third of their wealth (32%) on dementia care, compared with 11% for other diseases. African Americans, people with less than a high school education, and unmarried or widowed women faced the greatest economic burdens (see Figure 3).
The challenge, according to the researchers, is that “Medicare does not cover health-related expenses most valuable to those with chronic diseases or a life-limiting illness, such as homecare services, equipment, and non-rehabilitative nursing home care.” These costs are “largely borne by individuals and families, particularly among vulnerable subgroups.”26
Out-of-Pocket Expenditures on Dementia During the Last Five Years of Life as a Percentage of Wealth, by Population Subgroup
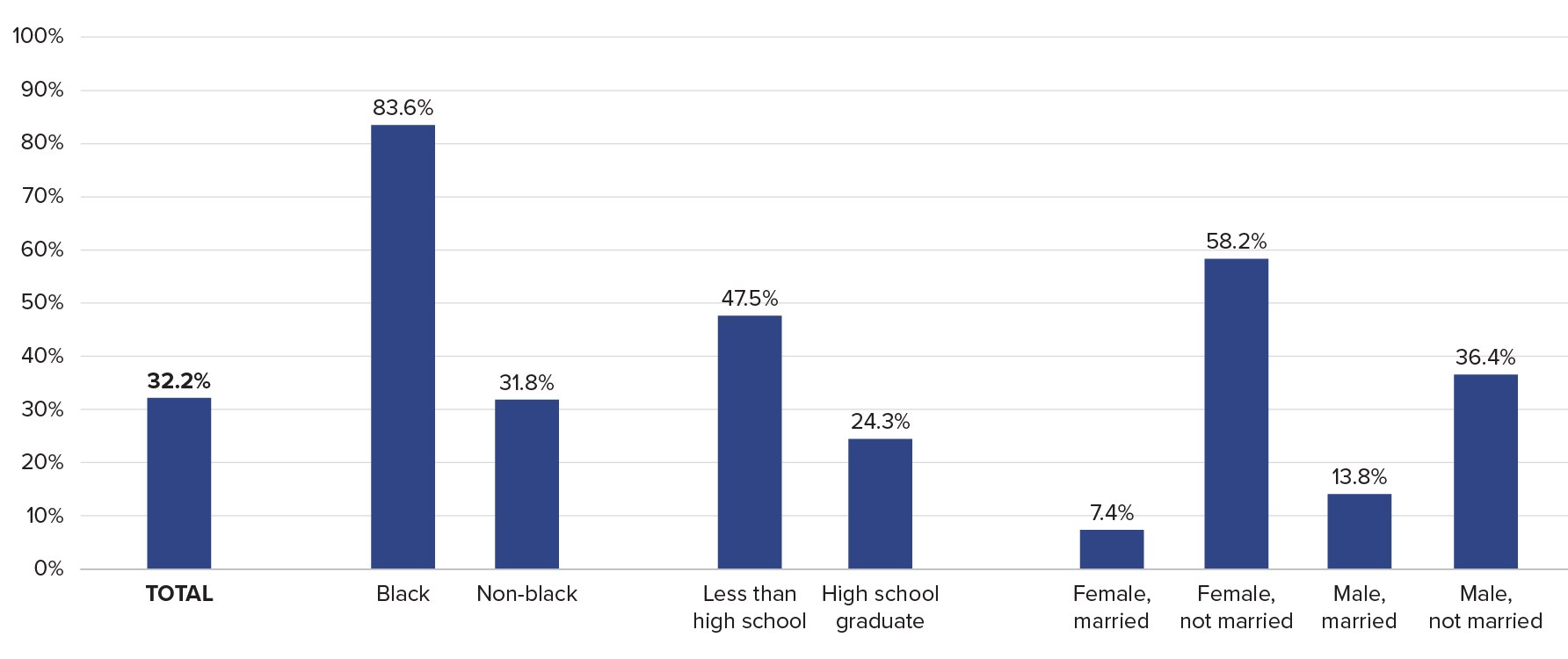
Source: Amy Kelley et al., “The Burden of Health Care Costs for Patients With Dementia in the Last Five Years of Life,” Annals of Internal Medicine 165, no. 3 (2015): 729-36.
When intangible costs are considered, caring for an older parent with dementia at home may be as costly as institutional care. Norma Coe and colleagues calculated the median direct and indirect costs to a daughter who cares for her mother who cannot be left alone.27 Their estimate accounted for not just a caregiver daughter’s lost earnings but also her future employability, lost leisure time, and impact on her well-being. Their findings suggest that costs total nearly $200,000 over two years, about the same as full-time care in a nursing facility.
Most U.S. adults ages 65 and older do not have long-term care insurance. In 2014, just 11% of older adults had such coverage, which protects them from catastrophic expenses related to long-term care support and dementia care if they did not qualify for Medicaid, analysis of HRS data showed.28 Caroline Pearson and colleagues estimated that by 2029, only about half of middle-income seniors ages 75 and older will have sufficient financial resources to pay for supportive care in a private residential setting (such as assisted living) if they develop conditions such as dementia.29
lthough older adults with probable dementia represent only about 10% of people ages 65 and older, they receive 41% of all care hours, and their informal caregivers make up one-third of all caregivers, according to data from the 2011 NHATS and the related National Study of Caregiving.30 Overall, daughters provide the bulk of unpaid care hours for people with dementia (39%), followed by spouses (25%), sons (17%), and other family and friends (20%). Older adults with dementia have larger caregiving networks than those without dementia and are twice as likely to have multiple caregivers sharing tasks.31
Caring for people with dementia living at home is the most time-intensive type of elder care. Among adults ages 65 and older who received help, those with probable dementia received many more hours of care, averaging 92 hours per month versus 68 for those without dementia.32 Caregiving was particularly time consuming for spouses or daughters or those who lived with the care recipient, totaling 145, 102, and 143 mean hours of caregiving per month, respectively.33
Another team of researchers found even larger differences in care for people with and without dementia, using data from the HRS and a somewhat different definition of care and age range.34 Among adults ages 70 and older, those with probable dementia received more than twice as many hours of monthly care on average than adults without dementia: 171 hours versus 66 hours.
Many people with dementia rely on family members even when they have paid care. Reckrey and colleagues showed that among those with probable dementia who lived at home and had paid care providers, informal family caregivers provided more than half the total weekly care hours (83 hours of paid and family care).35 In addition, Judith Kasper and colleagues found that family caregiving does not end when older adults with dementia move into residential care settings such as assisted living; about 80% of older adults with dementia living in residential care had at least one family or unpaid caregiver assisting with self-care or household activities.36
Unpaid caregivers for older adults with dementia report more difficulties than caregivers of those without dementia, but the differences have narrowed. Many of the circumstances of unpaid caregivers of older adults with dementia improved between 1999 and 2015, according to Jennifer Wolff and colleagues.37 They found that among primary family caregivers of older adults with dementia, the proportion reporting substantial physical or financial difficulty declined during the 16-year period, and their use of respite care nearly doubled.
The recent decline in the share of U.S. older adults with dementia is good news for families and health care providers. However, researchers question whether dementia prevalence can continue to decline in tandem with higher rates of obesity and diabetes, which are both cardiovascular risk factors for certain types of dementia.
Research has also identified large and growing disparities in dementia risk by socioeconomic status and race/ethnicity, which could slow progress in reducing dementia prevalence. The high rate of probable dementia among Latinos is of particular concern, given the increasing numbers of Latinos in the U.S. population.38 A better understanding of the causes of the recent decrease in dementia prevalence can help shape interventions that could contribute to further declines, with tremendous implications for older Americans, their families, and the costs of public programs. Policies that address growing disparities in dementia risk by ensuring equity of access to the resources and environments that contribute to healthy cognitive function are crucial to the overall health of the U.S. older population.39
Regardless of trends in dementia prevalence, rapid population aging in the United States will contribute to an increase in the number of people living with dementia in the coming decades. Demographers foresee shifting family structures as the large baby-boom generation (ages 56 to 74 in 2020) approaches ages when the risk of dementia increases. Declines in marriage, high rates of divorce, and lower fertility over the last few decades mean that more baby boomers may reach older ages without a spouse and with fewer adult children, on average, than earlier generations to rely on for care, suggests Emily Agree.40 However, the chances of having a partner still living in later life may increase, depending on mortality trends.
As the pool of traditional family caregivers for older Americans changes, patterns of family care for people with dementia and other functional difficulties may shift as well. Policymakers can help by making paid care more affordable and implementing policies that support the emerging roles of siblings, friends, cohabiting partners, and more distant relatives as caregivers.
Given that family caregivers “provide the lion’s share of care in the community,” Reckrey and colleagues call for expanding direct payments to family members who provide care. They also argue for “new ways of making paid caregiving more accessible throughout the income spectrum” via Medicaid expansion, increased spending on home- and community-based services, and tax credits or tax deductions for paid caregiving expenditures.41
1 Estimates of the number of people living with dementia varies depending on definitions and sources used; the Alzheimer’s Association estimates that 5.8 million Americans were living with Alzheimer’s dementia in 2019—most of them age 65 or older.
2 James E. Galvin et al., “The AD8:A Brief Informant Interview to Detect Dementia,” Neurology 65, no. 4 (2005): 559-64.
3 Judith D. Kasper et al., “The Disproportionate Impact of Dementia on Family and Unpaid Caregiving to Older Adults,” Health Affairs34, no. 10 (2015): 1642-49; and Judith D. Kasper, Vicki A. Freedman, and Brenda Spillman, “Classification of Persons by Dementia Status in the National Health and Aging Trends Study,” Johns Hopkins University School of Public Health, Technical Paper #5 (2013), https://www.nhats.org/scripts/documents/DementiaTechnicalPaperJuly_2_4_2013_10_23_15.pdf.
4 National Institute on Aging, “Alzheimer’s Disease Diagnostic Guidelines,” https://nia.nih.gov/health/alzheimers-disease-diagnostic-guidelines.
5 Krista L. Harrison et al., “Care Settings and Clinical Characteristics of Older Adults With Moderately Severe Dementia,” Journal of the American Geriatric Society 67, no. 9 (2019):1907-12; and Jennifer M. Reckrey et al., “Living in the Community With Dementia: Who Receives Paid Care?” Journal of the American Geriatric Society 68, no. 1 (2020): 186-91.
6 Eileen M. Crimmins et al., “Educational Differences in the Prevalence of Dementia and Life Expectancy With Dementia in the United States: Changes From 2000 to 2010,” Journals of Gerontology, Series B: Psychological Sciences and Social Sciences73, suppl. 1 (2019): S20-S28.
7 Kenneth M. Langa et al., “A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012,” JAMA Internal Medicine177, no. 1 (2017): 51-58. A separate study, using different methods, showed a more modest decline in dementia—from 12.0% in 2000 to 10.5% in 2012.
8 Vicki A. Freedman et al., “Short-Term Changes in the Prevalence of Probable Dementia: An Analysis of the 2011-2015 National Health and Aging Trends Study,” Journals of Gerontology, Series B: Psychological Sciences and Social Sciences73, suppl. 1 (2018): S48-S56.
9 Langa et al., “A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012.”
10 Freedman et al., “Short-Term Changes in the Prevalence of Probable Dementia.”
11 Freedman et al., “Short-Term Changes in the Prevalence of Probable Dementia.”
12 Heehyul Moon et al., “Dementia Prevalence in Older Adults: Variation by Race/Ethnicity and Immigrant Status,” American Journal of Geriatric Psychiatry 27, no. 3 (2019): 241-50.
13 Hui Liu et al., “Marital Status and Dementia: Evidence From the Health and Retirement Study,” Journals of Gerontology, Series B: Psychological Sciences and Social Sciences (2019).
14 Ellen A. Kramarow and Betzaida Tejada-Vera, “Dementia Mortality in the United States, 2000-2017,” National Vital Statistics Reports68, no. 2 (2019): 1-29.
15 Kramarow and Tejada-Vera, “Dementia Mortality in the United States, 2000-2017.”
16 This calculation assumes half of nursing home residents have dementia. Winnie Chi et al., “Community-Dwelling Older Adults With Dementia and Their Caregivers: Key Indicators From the National Health and Aging Trends Study” (Washington, DC: The Office of the Assistant Secretary for Planning and Evaluation, 2019), https://aspe.hhs.gov/system/files/pdf/260371/DemChartbook.pdf.
17 Judith D. Kasper et al., “The Disproportionate Impact of Dementia on Family and Unpaid Caregiving to Older Adults,” Health Affairs 34, no. 10 (2015): 1642-9.
18 Krista L. Harrison et al., “Care Settings and Clinical Characteristics of Older Adults With Moderately Severe Dementia,” Journal of the American Geriatric Society 67, no. 9 (2019): 1907-12.
19 Allison K. Gibson and Virginia E. Richardson, “Living Alone With Cognitive Impairment: Findings From the National Health and Aging Trends Study,” American Journal of Alzheimer’s Disease and Other Dementias 32, no. 1 (2017): 56-62.
20 Medicaid and CHIP Payment and Access Commission (MACPAC), “Home- and Community-Based Services,” http://www.macpac.gov/subtopic/home-and-community-based-services/.
21 Harrison et al., “Care Settings and Clinical Characteristics of Older Adults With Moderately Severe Dementia.”
22 Genworth, “Cost of Care Survey, 2019,” www.genworth.com/aging-and-you/finances/cost-of-care.html.
23 Jennifer M. Reckrey et al., “Living in the Community With Dementia: Who Receives Paid Care?” Journal of the American Geriatric Society 68, no. 1 (2020): 186-91.
24 Péter Hudomiet, Michael D. Hurd, and Susann Rohwedder, “The Relationship Between Lifetime Out-of-Pocket Medical Expenditures, Dementia, and Socioeconomic Status in the U.S.,” The Journal of the Economics of Ageing 14 (2019).
25 Amy S. Kelley et al., “The Burden of Health Care Costs for Patients With Dementia in the Last Five Years of Life,” Annals of Internal Medicine 163, no. 10 (2015): 729-36.
26 Kelley et al., “The Burden of Health Care Costs for Patients With Dementia in the Last Five Years of Life.”
27 Norma B. Coe, Meghan M. Skira, and Eric B. Larson, “A Comprehensive Measure of the Costs of Caring for a Parent: Differences According to Functional Status,” Journal of the American Geriatric Society 66, no. 10 (2018): 2003-8.
28 Richard W. Johnson, “Who Is Covered by Private Long-Term Care Insurance?” Urban Institute Brief, August 2, 2016, www.urban.org/research/publication/who-covered-private-long-term-care-insurance.
29 Caroline F. Pearson et al., “The Forgotten Middle: Many Middle-Income Seniors Will Have Insufficient Resources for Housing and Health Care,” Health Affairs 38, no. 5 (2019): 851-9.
30 Kasper et al., “The Disproportionate Impact of Dementia on Family and Unpaid Caregiving to Older Adults.”
31 Brenda C. Spillman et al., “Change Over Time in Caregiving Networks for Older Adults With and Without Dementia,” Journals of Gerontology, Series B: Psychological Sciences and Social Sciences (2019).
32 Kasper et al., “The Disproportionate Impact of Dementia on Family and Unpaid Caregiving to Older Adults.”
33 Kasper et al., “The Disproportionate Impact of Dementia on Family and Unpaid Caregiving to Older Adults.”
34 Esther M. Friedman et al., “U.S. Prevalence and Predictors of Informal Caregiving for Dementia,” Health Affairs 34, no. 10 (2015): 1637-41.
35 Reckrey et al., “Living in the Community With Dementia: Who Receives Paid Care?”
36 Kasper et al., “The Disproportionate Impact of Dementia on Family and Unpaid Caregiving to Older Adults.”
37 Jennifer L. Wolff et al., “Family Caregivers of Older Adults, 1999-2015: Trends in Characteristics, Circumstances, and Role-Related Appraisal,” Gerontologist 58, no. 6 (2018): 1021-32.
38 Freedman et al., “Short-Term Changes in the Prevalence of Probable Dementia.”
39 Langa et al., “A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012.”
40 Emily M. Agree, “Demography of Aging and the Family,” in Future Directions for the Demography of Aging: Proceedings of a Workshop (Washington, DC: The National Academies Press, 2018).
41 Reckrey et al., “Living in the Community With Dementia: Who Receives Paid Care?”

No other country in the world is experiencing population aging on the same scale as China.
The United Nations projects that there will be 366 million older Chinese adults by 2050, which is substantially larger than the current total U.S. population (331 million).1By that time, China’s share of adults ages 65 and older wills have risen from just 12% to a projected 26%. This rapid population aging—driven by recent declines in fertility and mortality—raises concerns about the health and well-being of older Chinese adults and will create considerable challenges for the health care system.
While life expectancy in China is increasing, older adults may spend more of their advanced years in poor health and with disabilities. Families have been the primary source of care for older adults, but the country’s rapid economic development and urbanization have separated millions of older adults from their children, contributing to an increasing demand for community-based health care.
These demographic and socioeconomic changes raise important questions for researchers and policymakers. How are older Chinese adults faring relative to their parents’ and grandparents’ generations? How is rapid urbanization affecting health and the availability of potential caregivers among older adults? How are older women faring relative to men, and which factors contribute to the gender gap in health? More broadly, what are the key factors associated with healthy aging in China, and what can policymakers do to improve health and reduce health disparities in the context of the country’s rapid socioeconomic development?
This issue of PRB’s Today’s Research on Aging (Issue 39) summarizes recent research on aging and health in China from U.S. National Institute of Aging-sponsored investigators and surveys, especially the China Health and Retirement Longitudinal Study (CHARLS) and Chinese Longitudinal Healthy Longevity Study (CLHLS). Results from these studies can shed light on the key determinants of healthy aging and help identify policies to address the challenges posed by rapid population aging in China.2The findings can also offer insights to policymakers in other countries with rapidly growing older populations.
China’s life expectancy has increased steadily during the past half century. In 1960, average life expectancy at birth in China was around 44 years. By 2017, it had increased to 76 years.3
Physical and cognitive health among older adults—especially women—is also improving with rising educational attainment and better medical care.4
Yi Zeng and colleagues find evidence of morbidity compression among China’s older adults—a reduction in the proportion of life spent with disability. Among adults ages 80 and older, mortality and self- reported disability rates have fallen relative to cohorts born 10 years earlier, according to their analysis of CLHLS data.5A recent study of adults ages 50 and older, based on CHARLS data, shows that at age 50, men can expect to live 24 years without activity limitations (26 years for women).6
These life expectancy gains and reductions in disability, however, are linked to rapid economic development in urban areas. Older adults in rural areas have not fared as well, leading to growing rural-urban disparities in health.7
Rising obesity rates and high smoking prevalence (among men) also present major health challenges for China’s aging population. In 2011, 28% of men and 38% of women ages 45 and older were overweight, putting them at higher risk of heart disorders, hypertension, diabetes, and stroke.8 Over half of men ages 45 and older (53%) smoked in 2011, compared with 5% of women in that age group.9 High levels of pollution—especially in urban areas—pose additional health risks.
“Public health campaigns and incentives are urgently needed on all these fronts so that the predictable long-term consequences of these behaviors on older age disease are not realized,” report researchers James Smith and his colleagues.9
Families have traditionally been the major source of financial and caregiving support for older adults in China, and most older adults have children living with them or nearby who can provide caregiving assistance. CHARLS data show that about 41% of older adults live with an adult child, and another 34% have an adult child living nearby.11
However, China’s relatively low fertility rate will reduce the availability of family caregivers in the future.12 Between 1980 and 2015, China applied a family planning policy limiting most families to only one child to control the country’s rapid growth. Adults ages 65 and older have five to six surviving children, on average, while younger cohorts born in the late 1950s and 1960s have fewer than two adult children on average.13 China’s total fertility rate (TFR), or average number of births per woman, is around 1.6. By comparison, the TFR in Asia as a whole (excluding China) is around 2.3, and the TFR in the United States is around 1.7.14
A growing number of young adults in China are also moving from rural to urban areas for employment opportunities. The share of the population living in urban areas increased from 19% in 1980 to 60% in 2019.15 This trend has left many older adults geographically separated from their adult children.16
Home- and community-based services could help fill the caregiving gap by providing older adults with medical, rehabilitation, and other healthcare services. These kinds of paid services are increasing but are not widely available, even in cities.17
As the demand for home-based care increases, the cost of providing that care will also increase with the rising number of disabled older adults. These costs include paid medical and nursing services, as well as opportunity costs for unpaid family members or friends who are providing care. Using CLHLS data, Zeng and colleagues find that older Chinese women are more likely than their male counterparts to become disabled. Yet expenditures on home-based care are lower for older women than men—an issue that needs more attention from policymakers, according to the study researchers.18
Chinese policymakers can promote healthy aging among older adults by implementing policies that address rural-urban disparities in health. Smith and colleagues argue that policies to help keep families together by allowing older adults to migrate to cities with their children could help reduce the caregiving gap in future years.19 Policymakers can also address older adults’ needs by expanding access to home- and community-based services, which older adults prefer over institutional (nursing home) care.20
Finally, the government could improve health and longevity by expanding access to health insurance coverage. By 2011, about 93% of Chinese adults ages 45 and older had health insurance. However, middle-aged and older adults with lower incomes were less likely to be insured—especially those with less education, divorced/widowed women, and those living in rural areas.21
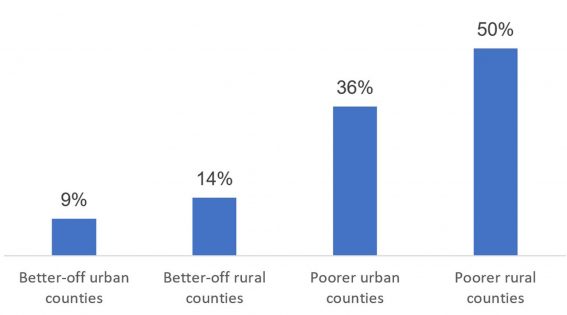
Older adults in rural China face additional health challenges. Self-reported health status varies widely across different areas of the country, with people from poorer rural counties reporting the worst health status. In the poorer rural counties, half of adults ages 45 and older reported being in poor health in 2011-2012, compared with 9% in better-off urban counties (see figure).22
Poor self-reported health in rural areas may reflect a lack of public health investments relative to better-off urban counties. Smith and colleagues find that living in areas in China without a strong public health infrastructure is associated with worse health in old age. In particular, using surface water instead of tap or underground water and using a toileting system without water may negatively affect health, including general health status and activities of daily living such as dressing, bathing, eating, and getting into or out of bed.23
Source: James P. Smith, Meng Tian, and Yaohui Zhao, “Community Effects on Elderly Health: Evidence from CHARLS National Baseline,” Journal of the Economics of Ageing 1-2 (2013): 50-59.
China’s rapid economic development has contributed to longer life expectancy, but it has also brought challenges related to lifestyle changes and pollution, particularly in urban areas.
Biological risk—measured biomarkers that reflect cardiovascular, metabolic, and inflammatory processes—is higher among older adults living in urban areas. This urban-rural gap in biological risk is largely explained by lifestyle factors such as lower levels of physical activity, according to a recent analysis of CHARLS data by Yuan Zhang and Eileen Crimmins.24 Another study of Chinese adults ages 65 and older, based on CHARLS data, showed that living in urban areas later in life is associated with better initial cognitive status but a faster rate of cognitive decline.25 The researchers argue that faster cognitive decline in cities may be linked to higher levels of population density and “constricted life space,” high housing costs, and the high cost of food and health services, among other factors.
China has also become one of the most polluted regions in the world, posing additional health risks for older adults—especially those living in cities. Long-term exposure to fine particulate matter in China is linked to greater risk of mortality in old age, while proximity to green space is associated with longer life expectancy.24 Older adults living in rural and southern areas are more sensitive to pollution than those residing in urban areas and in northern China, where pollution levels are higher. Higher sensitivity to pollutants in rural areas may be linked to time spent outdoors, health care access, baseline health status, or differences in the type of particulate matter present in rural and urban areas.26
Older Chinese women fare worse than men across a wide range of health measures. In 2011, women ages 45 and older were more likely than men to report being in poor or very poor health; experience depression, body pain, and hypertension; and have difficulties with activities of daily living (see table).
| Women (%) | Men (%) | |
| Poor or very poor health | 27.5 | 22.4 |
| High depressive symptoms | 40.7 | 28.1 |
| ADL/IADL difficulty | 29.6 | 21.8 |
| Body pain | 36.6 | 24.9 |
| Total hypertension | 43.9 | 40.3 |
| Undiagnosed hypertension | 39.6 | 42.8 |
| Get treatment if hypertensive | 48.8 | 44.6 |
Note: ADL means activities of daily living, such as dressing, bathing, eating, and getting into or out of bed. IADL means instrumental activities of daily living, such as shopping, cooking meals, managing money, and making phone calls.
Source: James P. Smith, John Strauss, and Yaohui Zhao, “Healthy Aging in China,” Journal of the Economics of Ageing 4, no. 2, (2014): 37-43.
Persistent gender gaps in education and literacy are partly to blame for older Chinese women’s poor health relative to men. Among adults ages 45 and older, about 40% of women were illiterate in 2011-2012, compared with 13% of men. Yet differences across age groups show the rapid progress women have made in recent years: The share of women ages 75 and older who were illiterate, at 80%, was 58 percentage points higher than the illiteracy rate among women ages 45 to 55 (22%).27
Researchers have also identified wide gaps in the cognitive abilities of older men and women. Gender differences in education largely explain this gap, especially among those in the oldest age groups and poorer communities. These gender differences in cognitive ability are decreasing as educational attainment increases among Chinese women in younger cohorts.29
Women’s childbearing patterns help explain the gender gap in health at older ages. Prior to the implementation of China’s one-child policy in 1980, women commonly had many children and started bearing them at a young age, which can negatively affect women’s health. Researchers have found that these effects can carry over into old age: Chinese women with four or more children are more likely to experience disabilities (impairment of activities of daily living, such as trouble dressing, bathing, eating, and getting into or out of bed) and poor self-rated health than women with one to three children.30
Conditions in childhood may also help explain gender gaps in health later in life. Using CLHLS data, Ke Shen and Yi Zeng find that favorable childhood conditions—based on birthplace, father’s socioeconomic status, and access to medical care in childhood—are linked to longer life expectancy through better socioeconomic status in adulthood.
“Public policies that target childhood well-being could effectively improve socioeconomic achievements in adulthood and, in turn, promote good health at senior ages,” argue researchers Shen and Zeng.31
However, this positive association is partially offset by “mortality selection.” The mortality selection hypothesis argues that unfavorable childhood circumstances result in high mortality rates among the most vulnerable people within a population and longer longevity for those who reach adulthood. This selection effect is larger for women than for men, possibly because surviving females tend to be healthier in countries with a strong son preference.32
“A girl might have worse nutrition and receive less care than a boy might. Such gender discrimination increases the mortality of vulnerable female infants, thus the surviving women are more selectively robust and have a higher chance of living into advanced ages,” according to the researchers.
China’s rapid economic development and urbanization may be a double-edged sword in their potential effects on the health and well-being of older adults. On the one hand, rapid economic growth has contributed to rising life expectancy and lower levels of disability. Rising educational attainment, especially among women, should lead to further improvements in health and reductions in health disparities among older adults. China’s health care system has also improved as the government has expanded access to public health services in both urban and rural areas.
On the other hand, rapid urbanization is separating millions of older adults from their adult children. Rising obesity rates, high smoking prevalence, and high levels of pollution also raise serious concerns about the health of China’s older adults in the coming decades.
These health challenges will be exacerbated by rapid demographic change. China has the world’s largest population of older adults and is experiencing population aging on an unprecedented—and unstoppable—scale.
China’s challenges will be shared by leaders in many other developing countries that have experienced rapid declines in fertility and mortality in recent decades. Studying the factors associated with healthy aging in China can help policymakers and planners address the looming shortage of caregivers and improve the health and well-being of older adults.
1 United Nations (UN), World Population Prospects 2019, https://population.un.org/wpp/DataQuery/.
2 Yi Zeng, “Towards Deeper Research and Better Policy for Healthy Aging—Using the Unique Data of Chinese Longitudinal Healthy Longevity Survey,” China Economic Journal, 5, no. 2-3 (2012): 131-49.
3 World Bank Open Data, https://data.worldbank.org/.
4 James P. Smith, John Strauss, and Yaohui Zhao, “Healthy Aging in China,” Journal of the Economics of Ageing 4, no. 2 (2014): 37-43.
5 Yi Zeng et al., “Improvements in Survival and Activities of Daily Living Despite Declines in Physical and Cognitive Functioning Among the Oldest-Old in China–Evidence From a Cohort Study,” Lancet 389, no. 10079 (2017): 1619-29.
6 Hao Luo et al., “Health Expectancies in Adults Aged 50 Years or Older in China,” Journal of Aging Health 28, no. 5 (2016): 758-74.
7 Zuyun Liu et al., “Are China’s Oldest-Old Living Longer With Less Disability? A Longitudinal Modeling Analysis of Birth Cohorts Born 10Years Apart,” BMC Medicine 17, no. 23 (2019).
8 Overweight is classified as having a body mass index of 25 or more.
9 Smith, Strauss, and Zhao, “Healthy Aging in China.”
10 Smith, Strauss, and Zhao, “Healthy Aging in China.”
11 Xiaoyan Lei et al., “Living Arrangements of the Elderly in China: Evidence From the CHARLS National Baseline,” China Economic Journal 8, no. 3 (2015): 191-214.
12 Smith, Strauss, and Zhao, “Healthy Aging in China.”
13 Yi Zeng, “Options of Fertility Policy Transition in China,” Population and Development Review 33, no. 2 (2007): 215-46.
14 Toshiko Kaneda, Charlotte Greenbaum, and Kaitlyn Patierno, 2019 World Population Data Sheet (Washington, DC: Population Reference Bureau, 2019).
15 UN, 2018 Revision of World Urbanization Prospects, https://population.un.org/wup/ (2018).
16 Smith, Strauss, and Zhao, “Healthy Aging in China.”
17 Zhanlian Feng et al., “China’s Rapidly Aging Population Creates Policy Challenges in Shaping a Viable Long-Term Care System,” Health Affairs 31, no. 12 (2012): 2764–73.
18 Yi Zeng et al., “Implications of Changes in Households and Living Arrangements for Future Home-Based Care Needs and Costs of Disabled Elders in China,” Journal of Aging Health 27, no. 3 (2015): 519-50.
19 Smith, Strauss, and Zhao, “Healthy Aging in China.”
20 Feng et al., “China’s Rapidly Aging Population Creates Policy Challenges in Shaping a Viable Long-Term Care System.”
21 Chuanchuan Zhang et al., “Health Insurance and Health Care Among the Mid-Aged and Older Chinese: Evidence From the National Baseline Survey of CHARLS,” Health Economics 26, no. 4 (2017): 431-49.
22 James P. Smith, Meng Tian, and Yaohui Zhao, “Community Effects on Elderly Health: Evidence From CHARLS National Baseline,” Journal of the Economics of Ageing 1-2 (2013): 50-9.
23 Smith, Tian, and Zhao, “Community Effects on Elderly Health: Evidence From CHARLS National Baseline.”
24 Yuan Zhang and Eileen Crimmins, “Urban-Rural Differentials in Age- Related Biological Risk Among Middle-Aged and Older Chinese,” International Journal of Public Health 64, no. 6 (2019): 831-39.
25 Yuanxi Xiang et al., “The Impact of Rural-Urban Community Settings on Cognitive Decline: Results From a Nationally Representative Sample of Seniors in China,” BMC Geriatrics 18, no. 1 (2018): 323.
26 John S. Ji et al., “Residential Greenness and Mortality in Oldest-Old Women and Men in China: A Longitudinal Cohort Study,” The Lancet Planetary Health 3, no. 1 (2019): e17-25.
27 Tiantian Li et al., “All-Cause Mortality Risk Associated With Long-Term Exposure to Ambient PM2·5 in China: A Cohort Study,” The Lancet Public Health 3, no. 10 (2018): e470-477.
28 Xiaoyan Lei et al., “Gender Differences in Cognition in China and Reasons for Change Over Time: Evidence From CHARLS,” Journal of the Economics of Ageing 1, no. 4 (2014): 46-55.
29 Lei et al., “Gender Differences in Cognition in China and Reasons for Change Over Time: Evidence From CHARLS.”
30 Xiaomin Li et al., “Female Fertility History and Mid-Late-Life Health: Findings From China,” Journal of Women and Aging 30, no. 1 (2018): 62-74.
31 Ke Shen and Yi Zeng, “Direct and Indirect Effects of Childhood Conditions on Survival and Health Among Male and Female Elderly in China,” Social Science & Medicine 119 (2014): 207-14.
32 Shen and Zeng, “Direct and Indirect Effects of Childhood Conditions on Survival and Health Among Male and Female Elderly in China.”

Sleep may be as important to health in old age as diet and exercise. Numerous studies have shown that sleeping too much or too little is associated with mortality among older adults.
A growing body of research indicates that not getting enough sleep may also increase the risk of several conditions and chronic diseases including diabetes, cardiovascular disease, obesity, and depression.
This issue of PRB’s Today’s Research on Aging (Issue 38) explores National Institute on Aging-supported research on sleep and aging, reviewing new evidence indicating that poor sleep may be both a sign of ill health and a trigger for processes related to disease and biological aging.
While sleep often tends to become more challenging for older people, insomnia—trouble falling asleep and staying asleep—is not a given with old age. The research examined here underscores the importance of screening for poor sleep and interventions that improve the sleep of older people.
Investigators are looking more deeply into the role of sleep in chronic disease and the aging process. Most studies on the relationship between sleep duration and health have been based on self-reported time spent asleep. These studies provide evidence of a U-shaped relationship between sleep duration and mortality: Regularly sleeping less than five hours daily or more than nine hours raises the risk of death.1
However, analysis of electronic sleep assessment data—gathered over multiple nights using wrist bands (actigraphy)—offers a more nuanced view. Diane Lauderdale of the University of Chicago and colleagues find sleeping less than six hours per night is associated with poor or fair health among older people but sleeping longer than average is not linked to any negative health consequences.2 Their study is based on sleep data for more than 700 adults ages 62 to 90 participating in the nationally representative National Social Life, Health, and Aging Project (NSHAP). As use of “actigraphy matures, our understanding of how sleep affects health may change,” they write.
A University of California, Los Angeles team finds that one night of partial sleep deprivation activates genes related to biological aging in older adults.3 For the study, 29 older adults between ages 61 and 86 spent four nights in a sleep laboratory. Following two uninterrupted nights of sleep, participants were not allowed to sleep between 11 p.m. and 3 a.m. and later awakened at 7 a.m. Researchers monitored their sleep and drew blood daily.
After a night partially deprived of sleep, participants’ blood showed signs of deterioration in the cell’s growth and division cycle. The researchers report that these findings “causally link sleep deprivation to the molecular processes associated with biological aging,” suggesting that insufficient sleep may increase the risk of chronic disease by “activating the molecular pathways that drive biological aging.”
Michael Irwin, Richard Olmstead, and Judith Carroll of the University of California, Los Angeles, find further evidence of the effect of sleep on the aging process by analyzing results from 72 distinct studies. This body of research—involving 50,000 participants in both clinical settings and the wider population—suggests that sleep disturbances (poor sleep or insomnia complaints) and long sleep duration (sleeping more than eight hours regularly) are related to increases in blood markers of inflammation.
Specifically, disturbed sleep and too much sleep are associated with the inflammation markers C-reactive protein (CRP) and interleukin-6 (IL-6). These markers tend to be related to chronic conditions such as diabetes and cardiovascular disease. Previous research shows that treating insomnia can reduce inflammation. The researchers argue that sleep disturbance and long sleep duration should be viewed as additional risk factors for inflammation that can be modified, like high-fat diets and sedentary lifestyles. For example, several studies show that insomnia treatments can reduce inflammation markers, offering evidence that sleep problems can be a cause of inflammation.4
Poor sleep is also related to depression in old age, according to several studies.5 A University of Michigan team finds disturbed sleep is associated with depression, regardless of the number of chronic medical conditions a participant has.6> The study tracked more than 3,500 older adults participating in the nationally representative Americans’ Changing Lives Study, which surveyed participants five times over 25 years.
The researchers show that older adults diagnosed with a higher number of chronic medical conditions–such as high blood pressure, diabetes, chronic lung disease, heart attack or other heart trouble, stroke, cancer, and arthritis—have higher levels of depressive symptoms. People sleeping poorly who also have heart trouble face a particularly high risk of having depressive symptoms.
In the researchers’ view, detecting sleep problems early and intervening with medications or behavioral change is crucial, and can have long-term benefits for physical and mental health. They point out that people with depression tend to use more health care services than average, and given high medical costs, early screening and treatment of disturbed sleep may reduce costs and have “enduring public health benefits.”
Severely disturbed sleep may be an early signal of impending dementia, a team of Canadian researchers show.7 Otherwise healthy older people may experience disturbed sleep, including severe insomnia and daytime sleepiness, prior to displaying other dementia-related symptoms, such as memory loss. For the study, the researchers examined the survey responses of more than 28,000 adults ages 50 and older collected through the Survey of Health, Ageing, and Retirement (SHARE) in 12 European countries.
Using data for participants with no symptoms of Alzheimer’s disease or dementia at the beginning of the study, researchers created a sleep disturbance index (based on measures of sleep problems, fatigue, use of sleep medication, trouble sleeping, and changes in sleep patterns). Analysis shows that each separate sleep measure is independently associated with a greater risk of Alzheimer’s disease, dementia, or death within four years. After accounting for overall health, high scores on the sleep disturbance index remain associated with a greater risk of developing dementia.
Dementia is known to profoundly disrupt the sleep-wake cycle of people with the disease and leave them highly active at night, creating a burden for their family caregivers. The researchers recommend that health care providers screen for sleep problems in older people, in order to detect dementia earlier and initiate interventions to potentially prevent or delay institutionalization.
Similarly, researchers at the University of California, Berkeley show that disrupted sleep related to Alzheimer’s disease may be different from or significantly more severe than typical age-related sleep impairment.8 Evaluating older people for sleep changes linked to Alzheimer’s, such as declines in non-rapid eye movement sleep, could be a potentially non-invasive way to identify individuals at risk for Alzheimer’s disease. They suggest that sleep impairment is both “a consequence and cause of the progression of Alzheimer’s disease; one that is modifiable, offering preventative and therapeutic treatment potential.”
Dementia-related brain changes may be linked to regularly sleeping less than six hours per night and may begin in middle age, researchers based in California, Pennsylvania, Alabama, Maryland, and Illinois find.9 More than 600 black and white adults (mean age 45) in the Coronary Artery Risk Development in Young Adults (CARDIA) study reported their typical sleep duration and then had brain MRIs five years later. Compared with those who slept between six and eight hours per night, the brains of short sleepers had a greater concentration of white matter hyperintensities (a hardening of arteries in the brain), which have been linked to stroke and vascular dementia.
Subscribe to our Today’s Research on Aging newsletter by sending an email to todaysresearch@prb.org with ‘Subscribe’ in the subject line.
[1] Francesco Cappuccio et al., “Sleep Duration and All-Cause Mortality: A Systematic Review and Meta-Analysis of Prospective Studies,” SLEEP 33, no. 5 (2010): 585-92.
[2] Diane Lauderdale et al., “Sleep Duration and Health Among Older Adults: Associations Vary by How Sleep Is Measured, Journal of Epidemiology and Community Health 70, no. 4 (2016): 361-6.
[3] Judith E. Carroll et al., “Partial Sleep Deprivation Activates the DNA Damage Response (DDR) and the Senescence-Associated Secretory Phenotype (SASP) in Aged Adult Humans,” Brain, Behavior, and Immunity 51, no. 1 (2016): 223-9.
[4] Michael R. Irwin et al., “Cognitive Behavioral Therapy and Tai Chi Reverse Cellular and Genomic Markers of Inflammation in Late-Life Insomnia: A Randomized Controlled Trial,” Biological Psychiatry 78, no. 1 (2015): 721-9; and Michael R. Irwin at al., “Cognitive Behavioral Therapy vs. Tai Chi for Late-Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial,” SLEEP 37, no. 1 (2014): 1543-52.
[5] Eun Lee et al., “Persistent Sleep Disturbance: A Risk Factor for Recurrent Depression in Community-Dwelling Older Adults,” SLEEP 36, no. 11 (2013): 1685-91.
[6] Amanda Leggett et al., “The Effect of Sleep Disturbance on the Association Between Chronic Medical Conditions and Depressive Symptoms Over Time,” Longitudinal and Life Course Studies 8, no. 2 (2017): 138-51.
[7] Roxanne Sterniczuk et al., “Sleep Disturbance Is Associated With Incident Dementia and Mortality,” Current Alzheimer Research 10, no. 7 (2013): 765-75.
[8] Bryce Mander et al., “Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease?” Neuron 39, no. 8 (2016): 552-6.
[9] Kristine Yaffe et al., “Sleep Duration and White Matter Quality in Middle-Aged Adults,” SLEEP 39, no. 9 (2016): 1743-47.

April 23, 2018
Senior Writer
First in a series of three articles excerpted from “Health and Working Past Traditional Retirement Ages,” Today’s Research on Aging, Issue 37.
By 2030, when the last of the large baby boom generation (born 1946 to 1964) has reached their mid-60s, more than 21 percent of the U.S. population is projected to be age 65 or older—up from about 15 percent in 2016.
The greying of America increases the costs of public programs for older adults and shifts the balance between working people supporting those programs and retirees receiving benefits. The old-age support ratio—the number of working-age adults ages 18 to 64 for every adult age 65 or older—is on course to shrink dramatically from 4.1 in 2016 to 2.8 by 2030. To relieve this fiscal pressure, policymakers continue to discuss new financial incentives to encourage people to postpone retirement, such as further raising the eligibility age for Social Security (currently age 67 for those born after 1960) and Medicare (now age 65).
Working longer can reduce public spending and enable some older workers to enter retirement with more financial security. Estimates based on past health trends suggest that most U.S. older adults can work an extra two years before retiring.1But a growing body of research suggests that baby boomers in their 50s and 60s are in poorer health—with more chronic disease and disability—than earlier generations at the same ages, potentially affecting their capacity to work longer.
Older adults ages 51 to 61 had a higher prevalence of six out of eight chronic conditions—including 37 percent higher diabetes prevalence—in 2004-2010 than their peers in 1992-1998, a 2016 study finds.2 Hiram Beltrán-Sánchez of the University of California-Los Angeles, Marsha Jiménez of Brown University, and S.V. Subramanian of Harvard University analyzed self-reported chronic disease in the 1990s and 2000s using data from the nationally representative U.S. Health and Retirement Study (HRS). Based on their findings, they argue that older adults nearing traditional retirement ages appear more burdened by health conditions than several decades ago.
In another recent study, a University of Southern California research team finds Americans are living longer with more disability. Eileen Crimmins, Yuan Zhang, and Yasuhiko Saito examined life expectancy trends and disability rates in the 40-year period from 1970 to 2010.3They show that the average total lifespan increased for both men and women, but so did the proportion of time spent living with a disability. For people ages 65 and older, they identify a “compression of morbidity”—that is, a reduction in the proportion of life spent with disability. However, people in their prime working years (ages 20 to 64) experienced increases in the proportion of life spent with a disability. The researchers argue that there is “little evidence” of improvements in health “that would support increasing the age at retirement.”
In addition, a 2012 study that synthesized the results of five nationally representative surveys finds increasing disability among those ages 55 to 64 between 2000 and 2008 (a group that included the oldest baby boomers).4 During the same period, disability levels continued to decline among the oldest Americans (ages 85 and older) and held steady among those ages 65 to 84.
Linda Martin of the RAND Corporation, and Robert Schoeni of the University of Michigan also document rising disability levels between 1997 and 2010 among middle-age and older Americans (ages 40 to 64).5 Their analysis, based on the nationally representative National Health Interview Survey data, identifies a link between increasing obesity and rising disability.
Martin and Schoeni take this line of research further, teaming up with HwaJung Choi of the University of Michigan for a 2016 study focusing on 55-to-69-year-olds using HRS data for 1998 to 2010.6 They find no improvement in levels of physical functioning and activity limitations during the period, and some evidence of worsening. They show that obese individuals face a greater likelihood of having physical limitations. Although baby boomers are less likely to smoke, have emphysema, or have heart attacks, they are more likely to be obese or have diabetes or high blood pressure than the previous generation at similar ages, they report.
Obesity is a risk factor for a variety of chronic conditions; it may also increase the likelihood of early retirement due to disability. Using HRS data, Francesco Renna and Nidhi Thakur of the University of Akron find that men and women under age 65 who were obese in 1992 were more likely to have a disability and retire early by 2002.7
“Obesity can largely impact labor market decisions directly through impairment of bodily functions and indirectly by being a risk factor for various diseases like hypertension, arthritis, etc.,” they write. About two in five Americans (43 percent) in their 40s and 50s were obese in 2015-2016, and thus face an increased risk of retiring early because of a disability or poor health.8
In a 2017 study, Choi and Schoeni examined trends in both physical limitations and cognitive impairment to compare the health of adults nearing retirement by generation.9 They find that adults in their late 50s today are in poorer health than their parents’ generation was at the same age, even though the younger group will have to work longer to collect full Social Security benefits.
For this study, they used HRS and National Health Interview Survey (NHIS) data and divided older Americans into five groups based on the age at which they are eligible to collect full Social Security retirement benefits: those born in 1937 or earlier (age 65); those born 1938 to 1942 (between ages 65 and 66); those born 1943 to 1954 (age 66); those born 1955 to 1959 (between ages 66 and 67), and those born in 1960 to 1962 (age 67).
They find that the younger groups had higher shares of people who had at least one limitation on their ability to perform a basic daily living task by themselves, such walking across a room, dressing, bathing, eating, and transferring into or out of bed (see figure).
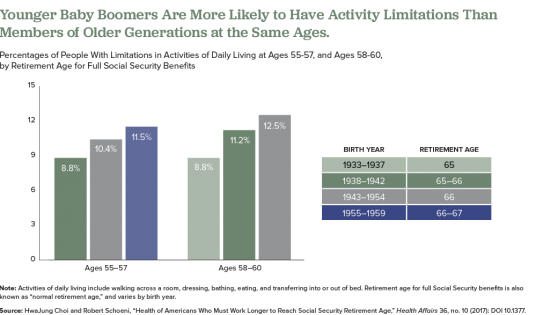
Also, they find that those born later tended to have higher rates of poor cognition, such as impaired memory and thinking ability, in their 50s compared with earlier generations at a similar age. Also, at age 50, people in the youngest group (born 1960 to 1962) were more likely to rate their own health as “fair” or “poor” than were people in the middle-three age groups when they were the same age, they report.
The researchers suggest that the increase of workers in their 50s and 60s who are in poor health will create significant challenges for them and their employers, including more people applying for Social Security disability payments. “Given the recent changes in health among the cohorts now approaching typical retirement age, further increases in the normal retirement age would place a substantial and disproportionate burden on these cohorts,” they argue.
The health of Americans in their 50s and early 60s today will shape labor force participation rates among the older population in the future. Understanding and monitoring these trends will be key as policymakers consider incentives to work longer and plan for future increases in the cost of public programs for older people.
Subscribe to our Today’s Research on Aging newsletter by sending an email to todaysresearch@prb.org with ‘Subscribe’ in the subject line.
1Courtney Coile, Kevin Milligan, and David Wise, “Health Capacity to Work at Older Ages: Evidence From the United States,” in Social Security Programs and Retirement Around the World: The Capacity to Work at Older Ages, National Bureau of Economic Research Conference Report, ed., David Wise (Chicago: University of Chicago Press, 2017).
2Hiram Beltrán-Sánchez, Marsha Jiménez, and S.V. Subramanian, “Assessing Morbidity Compression in Two Cohorts From the Health and Retirement Study,” Journal of Epidemiology and Community Health 70, no. 10 (2016): 1011-66.
3Eileen Crimmins, Yuan Zhang, and Yasuhiko Saito, “Trends Over Four Decades in Disability-Free Life Expectancy in the United States,” American Journal of Public Health 106, no. 7 (2016): 1287-93.
4Vicki Freedman et al., “Trends in Late-Life Activity Limitations in the United States: An Update From Five National Surveys,” Demography 49, no. 4 (2012).
5Linda G. Martin and Robert F. Schoeni, “Trends in Disability and Related Chronic Conditions Among the Forty-and-Over Population: 1997-2010,” Disability and Health Journal 7, no. 1 (2014): S4-14.
6HwaJung Choi, Robert Schoeni, and Linda G. Martin, “Are Functional and Activity Limitations Becoming More Prevalent among 55 to 69-Year-Olds in the United States?” PLoS One 11, no. 10 (2016): e0164565.
7Francesco Renna and Nidhi Thakur, “Direct and Indirect Effects of Obesity on U.S. Labor Market Outcomes of Older Working Age Adults,” Social Science & Medicine 71, no. 2 (2010): 405-13.
8National Center for Health Statistics (NCHS), “Prevalence of Obesity Among Adults and Youth: United States, 2015-2016” NCHS Data Brief, no. 288 (Oct. 2017).
9HwaJung Choi and Robert Schoeni, “Health of Americans Who Must Work Longer to Reach Social Security Retirement Age,” Health Affairs 36, no. 10 (2017): DOI 10.1377.
With Americans living longer and the large baby boom generation reaching ages 65 and beyond, the sheer numbers of people with conditions of old age—including Alzheimer’s disease and other dementias—are expected to rise dramatically in coming years. But there is some potentially good news: The share of the population with dementia may have fallen over the past 25 years—likely the result of better brain health related to more schooling and aggressive treatment of high blood pressure and diabetes.
This report explores the evidence of a decline in dementia and the trends that may shape the future prevalence of this debilitating condition—focusing on recent work by researchers supported by the National Institute on Aging (NIA).
It describes what we know (and do not know) about dementia patterns, examining known risk factors and vulnerable groups. This research can guide policymakers and public health professionals as they plan for an aging population and design strategies to address health and lifestyle factors related to dementia risk.
